Multiscale Models Bridging Molecular Signaling to Collective Behavior
Published:
I create hybrid models that embed intracellular signaling networks (GTPases like Rac/Rho) within cell-based simulations, enabling mechanistic connections between molecular processes and tissue-level patterns. These models track both cell positions and internal biochemical states using coupled ordinary and partial differential equations.
I consider models of intra-cellular signalling in cell polarization, that control how a cell determines its front and back. The objective is to uncover how cell-cell interactions, intracellular signalling, forces, and adhesion affect cell polarity and lead to the emergence of collective cell behaviour, in small to large cell groups.
The Multiscale Challenge
This challenge spans three orders of magnitude: GTPase signaling operates over seconds and micrometers, while tissue reorganization occurs over hours and millimeters. Current approaches face an impossible choice: cell-based models capture biological reality but resist mathematical analysis, while continuum models enable analytical insights but lose essential mechanisms.
My Hybrid PDE-Cell-Based Models
My models track 1D cells and their concentrations of intra-cellular proteins using reaction-diffusion equations (RDEs). The proteins Rac and Rho are known to regulate cell expansion and contraction. In my model, their concentrations determine the forces on the cell edges. Crucially my model accounts for single-cell experiment results.
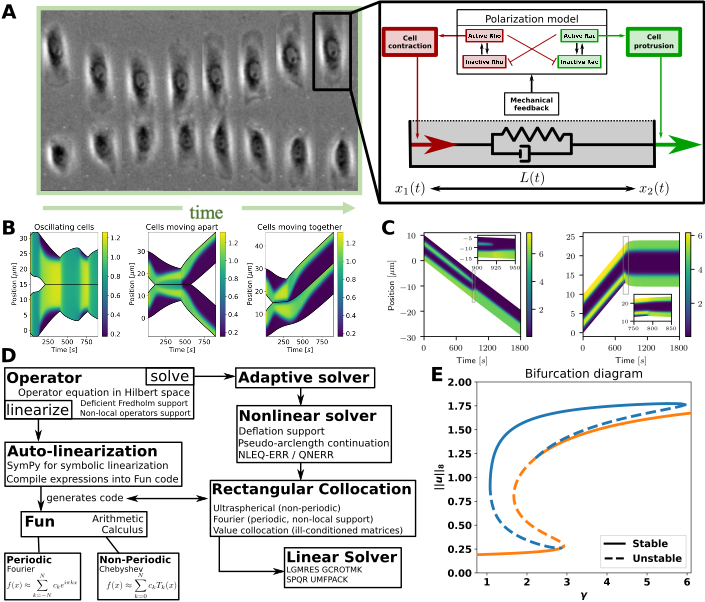
My cell repolarization / signalling model. A: Left: Cell collision experiment (Desai et al. 2013). Right: My mechanochemical model of a 1D cell has two modules: a polarization model, and visco-elastic “spring” connecting the cell’s edges. The polarization model describes intra-cellular proteins resulting in forces on the cell’s edges. B: Cell collisions in my model. C: Intra-cellular pattern formation determines cell behaviour. D: My modular ODE-iPDE analysis toolbox (based on NumPy). Symbolic linearization allows adoption to other spatial systems. E: Bifurcation diagram of a polarization model created by my toolbox. The “S-branch” consists of constant solutions, while the others are polarized states (see C).
Key Innovation: Mechanistic Rather Than Phenomenological
Rather than phenomenological rules, my models incorporate validated molecular mechanisms (receptor-ligand dynamics, cytoskeletal reorganization) and predict how perturbations to signaling cascades manifest in collective migration patterns—bridging three orders of magnitude from seconds/micrometers to hours/millimeters.
